AHR Target Analysis Report Summary


About the Target
The aryl hydrocarbon receptor (AHR) is a protein that plays a key role in various signaling pathways. It is found in the cytosol in an inactive form, forming a complex with other proteins such as heat shock protein 90 (HSP90), AHR-interacting protein (AIP), p23, and c-SRC. However, when AHR agonists bind to it, the conformation of AHR changes, leading to its translocation to the nucleus. In the nucleus, AHR interacts with ARNT and together they regulate the transcription of genes containing xenobiotic responsive elements (XREs)[1].
AHR has been found to have important functions in different cell types. In Tr1 cells, it collaborates with c-Maf and STAT3 to induce the expression of IL-10, IL-21, and CD39. It also controls the metabolic reprogramming of Tr1 cells by regulating the expression of enzymes involved in glucose metabolism. In Th17 cells, AHR works with RORgammat to induce IL-22 expression. Additionally, AHR inhibits STAT1 and STAT5 activation and reduces IL-2 expression by inducing the epigenetic modifier Aiolos in cooperation with STAT3. Furthermore, AHR contributes to the plasticity of Th17 cells by promoting their trans-differentiation into Tr1 cells[2].
AHR has been implicated in the regulation of cytokines and repair mechanisms in the intestinal tract. It can be activated by small molecules that diffuse across the mucus lining and bind to AHR. On the other hand, larger molecules like TLR4 ligands, such as lipopolysaccharide, cannot diffuse unless there is a breach in the mucus lining. The presence of both AHR activators and TLR4 ligands leads to robust cytokine and chemokine production and aids in the repair response[3].
Another important aspect of AHR is its involvement in melanoma resistance to BRAFi treatment. AHR activation, in cooperation with alpha-ligands, drives the resistance genes in melanoma cells, leading to melanoma relapse. In drug-resistant cells, endogenous alpha-ligands activate the AhR/ARNT pathway, promoting the alpha-signature and driving the expression of BRAFi-resistance genes. Loss-of-function experiments further confirmed the dependency of these signatures on AhR and ARNT[4].
Lastly, AHR has molecular effects on endothelial cells and endothelial progenitor cells. It activates the AhR genomic pathway and induces inflammatory pathways, leading to the production of proteins involved in inflammation, thrombosis, and leukocyte adhesion. It also promotes endothelial progenitor cell senescence, reducing their proliferation. These effects are mediated through various pathways, including the activation of nuclear factor kappaB (NF-kappaB), activating protein 1 (AP-1), and the production of reactive oxygen species (ROS)[5].
In summary, AHR is a versatile protein that plays a vital role in various signaling pathways and cellular processes. It is involved in transcriptional regulation, metabolic reprogramming, immune responses, and the development of drug resistance in melanoma. Its activation triggers a cascade of events leading to the modulation of gene expression and cellular functions.
Based on the provided context information, here are the key viewpoints regarding the protective role of AhR:
In obese non-diabetic individuals with high small intestinal inflammation scores, lower AhR activity was observed, indicating a potential link between AhR and intestinal inflammation [6].
Activation of AhR in intestinal epithelial cells improved barrier integrity, reduced paracellular permeability, and prevented the expression of pro-inflammatory cytokine IL-8, suggesting the protective role of AhR in maintaining intestinal barrier function [6].
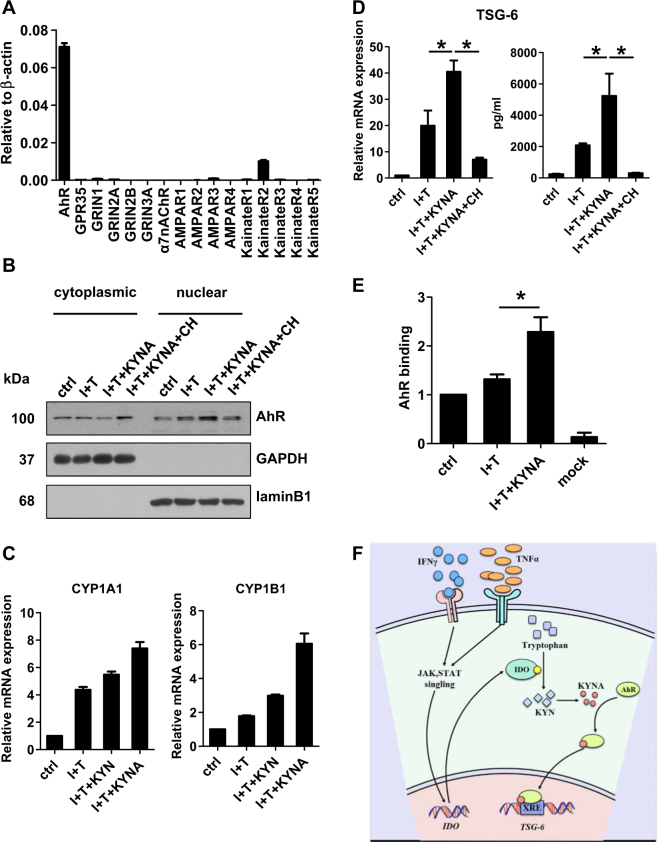
KYNA, an IDO metabolite, activated AhR signaling in human MSCs, leading to increased translocation of AhR to the nucleus and upregulation of AhR-regulated enzymes CYP1A1 and CYP1B1 [7].
Addition of a specific AhR antagonist abolished the enhanced production of TSG-6, indicating that AhR activation is necessary for promoting TSG-6 expression [7].
AhR was found to bind to the TSG-6 promoter region, and KYNA treatment enhanced this binding capability, suggesting that KYNA activates AhR to directly promote TSG-6 expression by binding to its promoter [7].
KYNA was effective at lower concentrations compared to another IDO metabolite, KYN, in inducing AhR-responsive genes in human MSCs [7].
Overall, these findings highlight the protective role of AhR in intestinal barrier function and TSG-6 expression, indicating its potential therapeutic significance in managing intestinal inflammation and immune cell infiltration [6][7].
Figure [1]

Figure [2]

Figure [3]
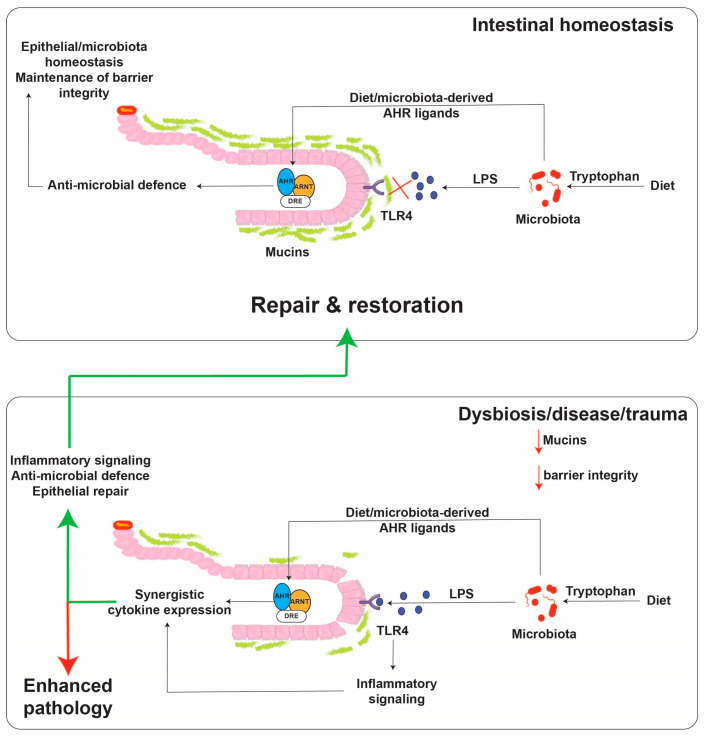
Figure [4]
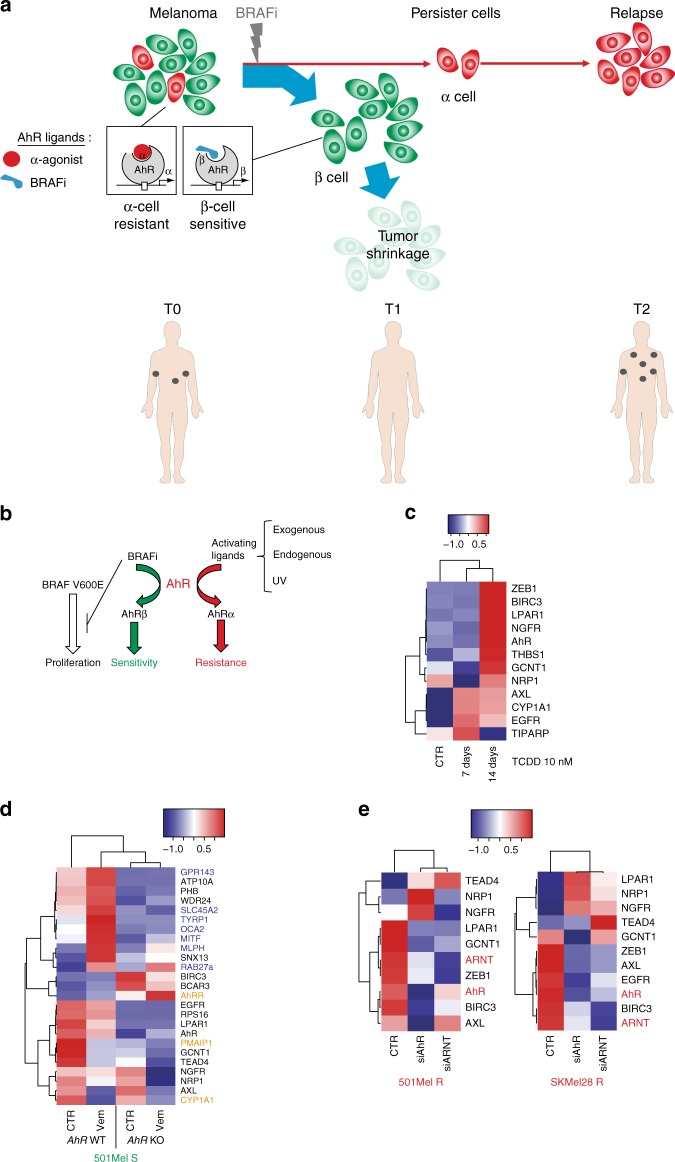
Figure [5]
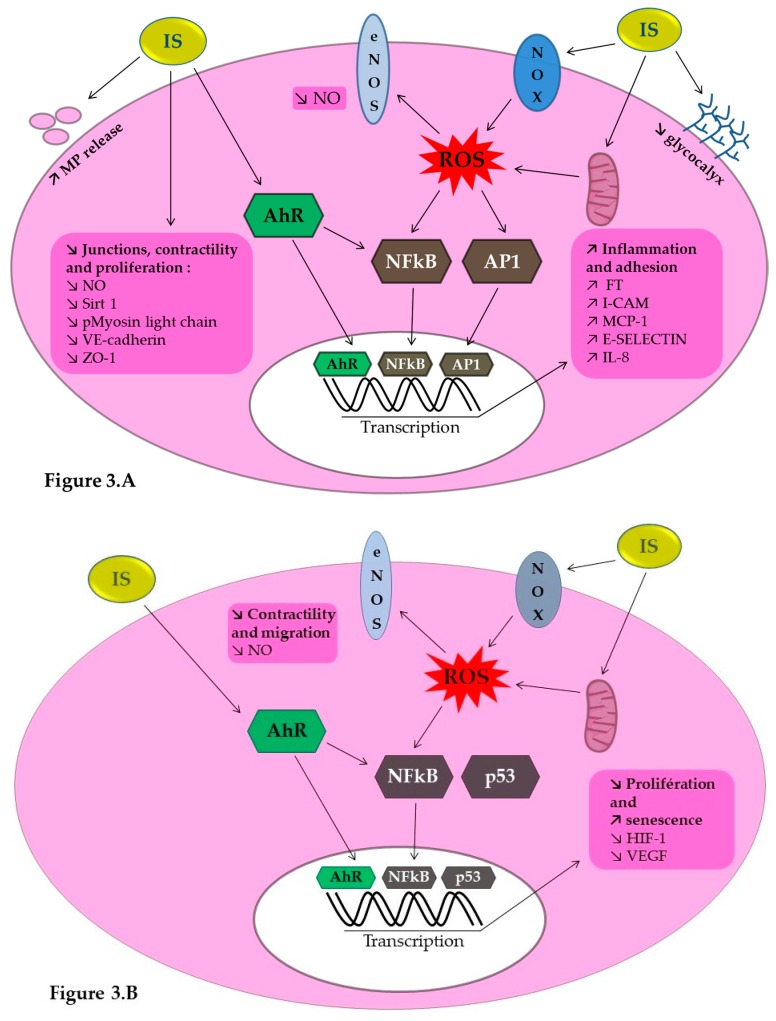
Figure [6]
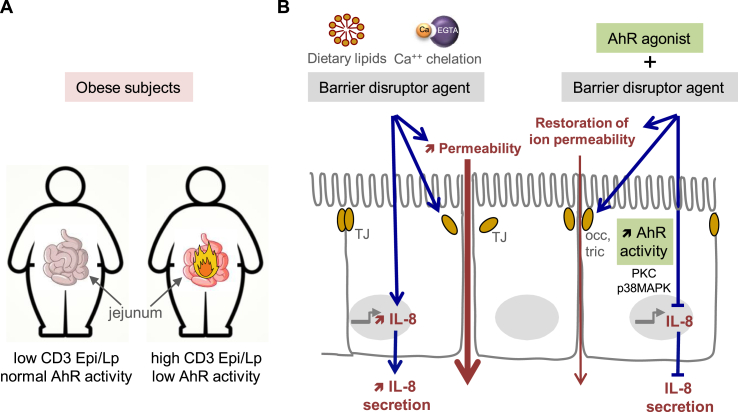
Figure [7]
Note: If you are interested in the full version of this target analysis report, or if you'd like to learn how our AI-powered BDE-Chem can design therapeutic molecules to interact with the AHR target at a cost 90% lower than traditional approaches, please feel free to contact us at BD@silexon.ai.
More Common Targets
ABCB1 | ABCG2 | ACE2 | AHR | AKT1 | ALK | AR | ATM | BAX | BCL2 | BCL2L1 | BECN1 | BRAF | BRCA1 | CAMP | CASP3 | CASP9 | CCL5 | CCND1 | CD274 | CD4 | CD8A | CDH1 | CDKN1A | CDKN2A | CREB1 | CXCL8 | CXCR4 | DNMT1 | EGF | EGFR | EP300 | ERBB2 | EREG | ESR1 | EZH2 | FN1 | FOXO3 | HDAC9 | HGF | HMGB1 | HSP90AA1 | HSPA4 | HSPA5 | IDO1 | IFNA1 | IGF1 | IGF1R | IL17A | IL6 | INS | JUN | KRAS | MAPK1 | MAPK14 | MAPK3 | MAPK8 | MAPT | MCL1 | MDM2 | MET | MMP9 | MTOR | MYC | NFE2L2 | NLRP3 | NOTCH1 | PARP1 | PCNA | PDCD1 | PLK1 | PRKAA1 | PRKAA2 | PTEN | PTGS2 | PTK2 | RELA | SIRT1 | SLTM | SMAD4 | SOD1 | SQSTM1 | SRC | STAT1 | STAT3 | STAT5A | TAK1 | TERT | TLR4 | TNF | TP53 | TXN | VEGFA | YAP1

