PRKAA1 Target Analysis Report Summary


About the Target
AMPK, also known as PRKAA1, is a key regulator of various cellular processes. In the fed state, AMPK inhibits autophagy by activating mTORC1 [1A]. Conversely, during glucose starvation, AMPK is activated and causes mTORC1 to dissociate from lysosomes, thereby reducing its activity [1B]. AMPK activation plays a crucial role in regulating metabolism, protein transportation, transcription factors, and enzymes [2]. It enhances substrate uptake and utilization, promotes mitochondrial biogenesis, and exerts a cardioprotective function [2]. AMPK is also involved in mediating multiple physiological signal pathways [2]. Exercise and exercise-mimetics, such as AICAR, Metformin, and Resveratrol, activate AMPK and positively regulate pathways related to endurance capacity, fat metabolism, and mitochondrial biogenesis [3]. Furthermore, AMPK activation has been linked to vasodilation, angiogenesis, and increased BDNF expression [3]. In conditions of starvation, AMPK activation promotes survival through the inhibition of protein translation, increased UPS activity, and modulation of proteasome and proteostasis genes [4]. AMPK activation is influenced by the AMP/ATP ratio, upstream kinase activity (e.g., LKB1, CaMKKbeta), and upstream phosphatase activity [5]. Once activated, AMPK stimulates catabolic pathways to increase ATP levels and suppresses anabolic pathways [5]. AMPK is considered a central regulator of energy metabolism [5].
Based on the given context information and the keyword "AMPK" (synonymous with PRKAA1), several key viewpoints can be summarized:
AMPK inhibits mTORC1 in response to glucose starvation through different mechanisms, including the phosphorylation and activation of the mTOR negative regulator tuberous sclerosis complex 2 (TSC2) and the phosphorylation and inhibition of the mTORC1 component regulatory-associated protein of mTOR (Raptor) [6].
Glucose restriction leads to the induction of GADD34 by ATF4, which binds and dephosphorylates TSC2, resulting in mTORC1 inhibition [6].
TBC1D7, an additional component of the TSC complex, plays a role in the activation of mTORC1 when depleted [6].
Cells can adapt to oligomycin treatment by undergoing persistent oscillations, stable adaptation at a lower level of ATP, or stable adaptation at a high level of ATP [7].
The addition of oligomycin blocks ATP production by oxidative phosphorylation, leading to a decrease in ATP levels and an increase in AMPK activity [7].
Positive feedback regulation increases the rate of flux through glycolysis, subsequently increasing ATP production and reducing AMPK activity [7].
Negative feedback regulation of glycolysis is triggered by high ATP levels and citrate buildup in the TCA cycle, leading to a reduction in glycolytic flux [7].
Insulin-treated cells, in the presence of high glucose and glutamine, maintain a high level of glycolytic flux and ATP production, even in the absence of oxidative phosphorylation [7].
Metformin inhibits mTOR signaling and leads to changes in gene expression in primary hepatocytes [8].
10. Suberoylanilide hydroxamic acid treatment affects multiple biological functions and pathways, including the inhibition of AMPK signaling [9].
11. eGSM (an experimental compound) can induce autophagy by either activating AMPK or suppressing mTOR activity [10].
12. eGSM activates AMPK but not mTOR activity in A549 cells, while it suppresses mTOR but not AMPK activity in SNU2292 cells [10].
13. The metabolization of eGSM in A549 cells generates a metabolite that activates AMPK, contributing to the induction of autophagy [10].
Note: The references [number] correspond to the numbered context snippets from where the information was extracted.
Figure [1]

Figure [2]

Figure [3]

Figure [4]

Figure [5]
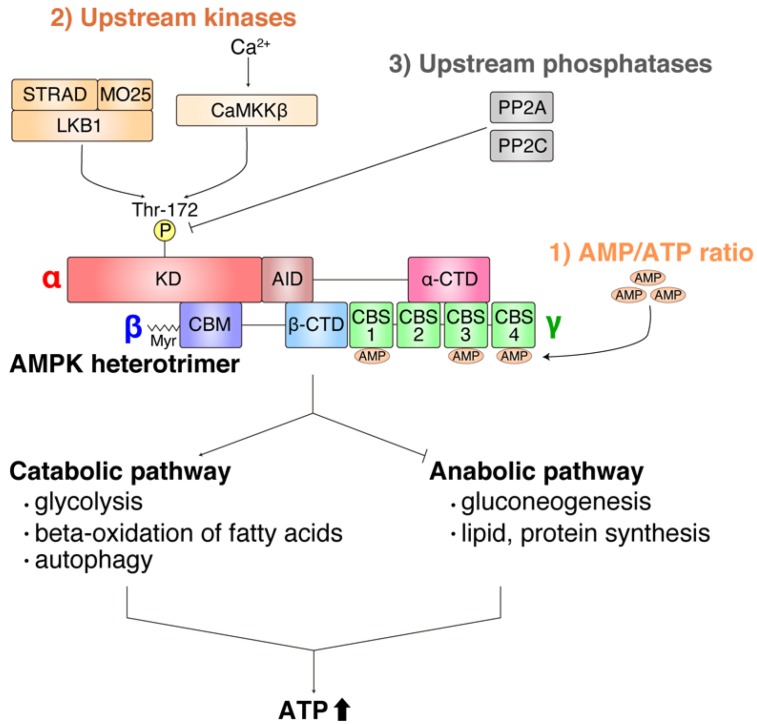
Figure [6]

Figure [7]

Figure [8]

Figure [9]

Figure [10]

Note: If you are interested in the full version of this target analysis report, or if you'd like to learn how our AI-powered BDE-Chem can design therapeutic molecules to interact with the PRKAA1 target at a cost 90% lower than traditional approaches, please feel free to contact us at BD@silexon.ai.
More Common Targets
ABCB1 | ABCG2 | ACE2 | AHR | AKT1 | ALK | AR | ATM | BAX | BCL2 | BCL2L1 | BECN1 | BRAF | BRCA1 | CAMP | CASP3 | CASP9 | CCL5 | CCND1 | CD274 | CD4 | CD8A | CDH1 | CDKN1A | CDKN2A | CREB1 | CXCL8 | CXCR4 | DNMT1 | EGF | EGFR | EP300 | ERBB2 | EREG | ESR1 | EZH2 | FN1 | FOXO3 | HDAC9 | HGF | HMGB1 | HSP90AA1 | HSPA4 | HSPA5 | IDO1 | IFNA1 | IGF1 | IGF1R | IL17A | IL6 | INS | JUN | KRAS | MAPK1 | MAPK14 | MAPK3 | MAPK8 | MAPT | MCL1 | MDM2 | MET | MMP9 | MTOR | MYC | NFE2L2 | NLRP3 | NOTCH1 | PARP1 | PCNA | PDCD1 | PLK1 | PRKAA1 | PRKAA2 | PTEN | PTGS2 | PTK2 | RELA | SIRT1 | SLTM | SMAD4 | SOD1 | SQSTM1 | SRC | STAT1 | STAT3 | STAT5A | TAK1 | TERT | TLR4 | TNF | TP53 | TXN | VEGFA | YAP1

