PARP1 Target Analysis Report Summary


About the Target
PARP1, also known as poly-(ADP)-ribose polymerase, plays a crucial role in the DNA damage response [1]. It is involved in the repair of oxidative DNA damages [3] and is activated by DNA damage [2]. PARP1 detects DNA damage through its DNA-binding domain (DBD) and synthesizes poly(ADP) ribose (pADPr) on acceptor proteins, leading to the recruitment of repair proteins to the damaged DNA [2]. It is involved in various repair mechanisms, including base excision repair (BER), homologous recombination (HR), and alternative non-homologous end joining (Alt-NHEJ) [3]. PARP1 inhibitors prevent the synthesis of pADPr and hinder downstream repair processes, increasing the duration of DNA lesions [2]. The principle of synthetic lethality applies to PARP inhibitors, as they selectively kill tumor cells by inducing DNA damage that cannot be repaired in the absence of a functional HR pathway [4]. PARP inhibition also leads to mitotic catastrophe and DNA replication stress [5]. In summary, PARP1 is a key protein involved in DNA damage response and repair mechanisms, and its inhibition has significant implications for cancer treatment.
PARP1, also known as PARP, plays a crucial role in various biological processes and is associated with different diseases. In breast cancer cells, the combination of PARP inhibitors with zoledronic acid disrupts antiapoptotic and proliferative signaling pathways, leading to hindered cell division [6]. PARP1 is also involved in regulating gene expression and can act as a transcriptional coactivator/co-repressor in the inflammatory process [7]. Moreover, PARP1 activity influences the function of insulators and may be a target for treating EBV infection [7]. In cardiovascular diseases like atrial fibrillation (AF) and atherosclerotic CAD, PARP1 activation and NAD+ depletion contribute to ATP depletion and ROS production, leading to mitochondrial dysfunction [9]. Additionally, PARP1 inhibitors have shown antiviral effects, potentially inhibiting viral replication and modulating inflammation [10]. The wide range of PARP1 targets and its involvement in various diseases suggest that targeting PARP1 could have therapeutic benefits in different contexts [8] [9].
Figure [1]

Figure [2]

Figure [3]

Figure [4]

Figure [5]
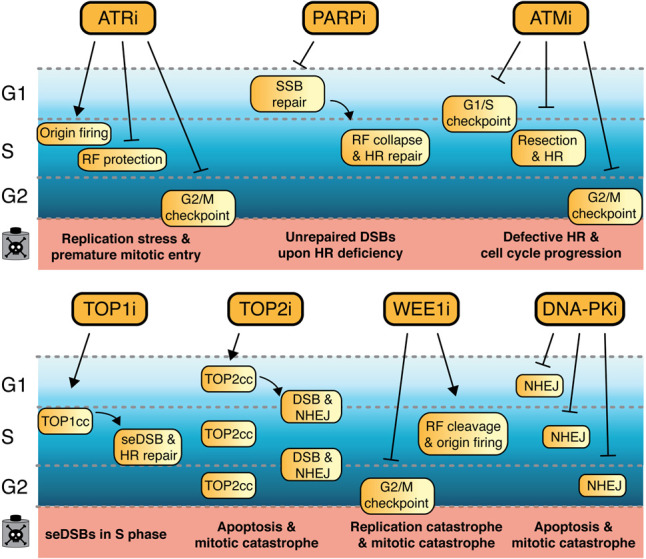
Figure [6]

Figure [7]

Figure [8]

Figure [9]

Figure [10]

Note: If you are interested in the full version of this target analysis report, or if you'd like to learn how our AI-powered BDE-Chem can design therapeutic molecules to interact with the PARP1 target at a cost 90% lower than traditional approaches, please feel free to contact us at BD@silexon.ai.
More Common Targets
ABCB1 | ABCG2 | ACE2 | AHR | AKT1 | ALK | AR | ATM | BAX | BCL2 | BCL2L1 | BECN1 | BRAF | BRCA1 | CAMP | CASP3 | CASP9 | CCL5 | CCND1 | CD274 | CD4 | CD8A | CDH1 | CDKN1A | CDKN2A | CREB1 | CXCL8 | CXCR4 | DNMT1 | EGF | EGFR | EP300 | ERBB2 | EREG | ESR1 | EZH2 | FN1 | FOXO3 | HDAC9 | HGF | HMGB1 | HSP90AA1 | HSPA4 | HSPA5 | IDO1 | IFNA1 | IGF1 | IGF1R | IL17A | IL6 | INS | JUN | KRAS | MAPK1 | MAPK14 | MAPK3 | MAPK8 | MAPT | MCL1 | MDM2 | MET | MMP9 | MTOR | MYC | NFE2L2 | NLRP3 | NOTCH1 | PARP1 | PCNA | PDCD1 | PLK1 | PRKAA1 | PRKAA2 | PTEN | PTGS2 | PTK2 | RELA | SIRT1 | SLTM | SMAD4 | SOD1 | SQSTM1 | SRC | STAT1 | STAT3 | STAT5A | TAK1 | TERT | TLR4 | TNF | TP53 | TXN | VEGFA | YAP1

