INS Target Analysis Report Summary


About the Target
Insulin, or INS, plays a crucial role in various biological processes, and its dysfunction can lead to several health conditions. The key viewpoints identified in the provided context are:
Insulin secretion and beta-cell dedifferentiation: Metabolic stress, particularly oxidative stress and proinflammatory cytokines, contribute to the dedifferentiation of beta cells. The renin-angiotensin system (RAS) activation is involved in inducing this metabolic stress [1]. Suppression of NF-kappaB signaling can alleviate the dedifferentiation effect on beta cells, leading to increased insulin secretion. Furthermore, RAS inhibitors have demonstrated a similar effect on beta cells [1].
Insulin and progression of non-alcoholic fatty liver disease (NAFLD): Various pathogenetic mechanisms contribute to NAFLD progression. High caloric intake, gut dysbiosis, and adipose tissue enlargement are factors that influence the gut-liver axis. Genetic variants, such as PNPLA3 and GCKR, impact adipose tissue lipolysis and de novo lipogenesis (DNL) [2]. Therapeutic interventions focus on augmenting fatty acid oxidation and targeting DNL through various pharmacological agents [2].
Insulin's role in follicle growth and atresia: Insulin levels and LH stimulation influence theca cell androgen production in polycystic ovary syndrome (PCOS). Elevated LH and insulin levels enhance the expression of specific genes, such as aromatase and LH receptor, contributing to premature differentiation of granulosa cells [3].
Integration of metabolism and immune responses: Obesity and saturated fatty acids (SFA) promote a pro-inflammatory macrophage phenotype, favoring glycolysis for ATP generation. This leads to TCA cycle fragmentation and increased accumulation of succinate and citrate, which negatively affect insulin sensitivity [4]. Conversely, monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA) drive an anti-inflammatory phenotype and promote oxidative phosphorylation, fatty acid oxidation, and glutamine metabolism [4].
Protective effects of compound 9c against ER stress in beta cells: Endoplasmic reticulum (ER) stress triggers a response known as the unfolded protein response (UPR), which can lead to beta cell dysfunction and apoptosis. Compound 9c has shown protective effects by down-regulating UPR components and up-regulating PDX1, MAFA, and insulin genes [5].
In conclusion, these viewpoints highlight the importance of insulin and its involvement in various physiological processes, including beta cell function, liver metabolism, ovarian follicle growth, immune responses, and ER stress. Further research in these areas may provide insights into the development of therapeutic interventions for related health conditions.
Based on the provided context information, here is a comprehensive summary based on the query keywords:
Insulin is a hormone involved in regulating glucose metabolism in the body. In beta cells, immature cells show elevated insulin secretion in basal conditions but have reduced ability to respond to increases in glucose levels. Mature beta cells, on the other hand, have lower basal insulin secretion but respond to increased glucose levels with an increased insulin secretion [6]. The expression of synaptotagmin 4 (Syt4), a vesicle protein, promotes functional beta cell maturation by reducing insulin vesicle sensitivity to calcium influx, which leads to a more efficient insulin secretory response [6].
In the context of probiotics, incorporating spores of the bacterium B. subtilis in the diet can result in the formation of a beneficial biofilm in the host intestine. This biofilm produces beneficial molecules such as nitric oxide (NO) and colony-stimulating factors (CSF) that influence longevity. These molecules downregulate the insulin receptor, activating longevity-regulating genes and promoting resistance to age-related diseases [7].
When it comes to liver transplant patients, certain immunosuppressive drugs used post-transplantation can contribute to the development of post-transplant diabetes mellitus (PLTDM). Calcineurin inhibitors, a type of immunosuppressive drug, inhibit key transcription factors involved in beta-cell survival and reduce glucose transporter expression and mitochondrial activity, thus impacting insulin production and glucose uptake. Corticosteroids, another class of immunosuppressive drugs, impair insulin action and result in deficient exocytosis of insulin and eventually apoptosis [8].
In a study focusing on the insulin signaling pathway, Spearman correlations between proximal signaling proteins of interest provide insight into the relationship between these proteins. However, specific findings from this study are not provided in the given context [9].
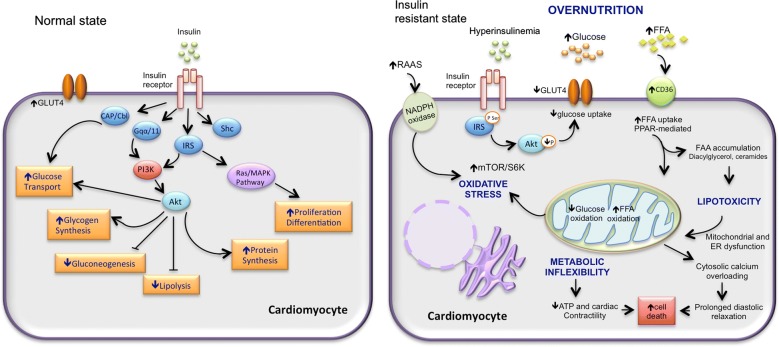
Diabetic cardiomyopathy, a complication of diabetes, involves insulin resistance and metabolic derangements. Insulin resistance leads to high lipid oxidation and low glucose oxidation. Activation of the renin-angiotensin-aldosterone system (RAAS) can further contribute to mitochondrial dysfunction, endoplasmic reticulum stress, oxidative stress, abnormal calcium handling, low ATP production, and eventually cardiomyocyte death [10].
In summary, the role of insulin varies across different contexts. It is involved in regulating glucose metabolism in beta cells and is crucial for insulin secretion in response to glucose levels. Probiotics can influence insulin signaling and impact longevity. Immunosuppressive drugs used in liver transplant patients can disrupt insulin-related processes and contribute to diabetes. The insulin signaling pathway itself is influenced by various proteins, although specific findings were not provided. Insulin resistance and metabolic derangements play a role in the development of diabetic cardiomyopathy.
Figure [1]
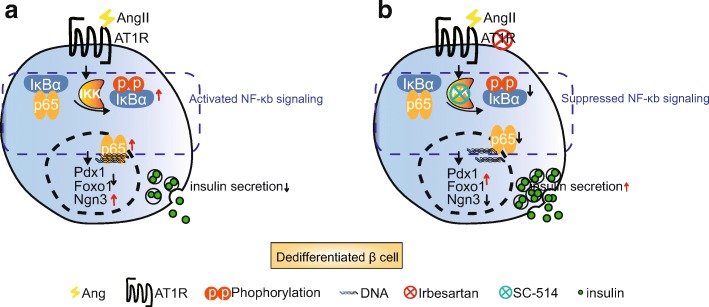
Figure [2]
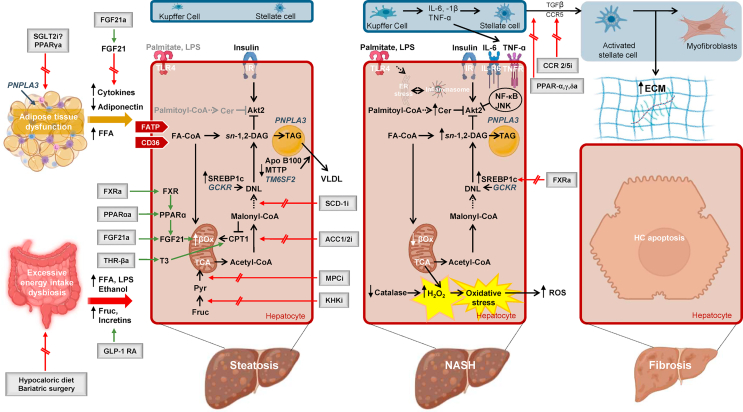
Figure [3]

Figure [4]

Figure [5]

Figure [6]

Figure [7]
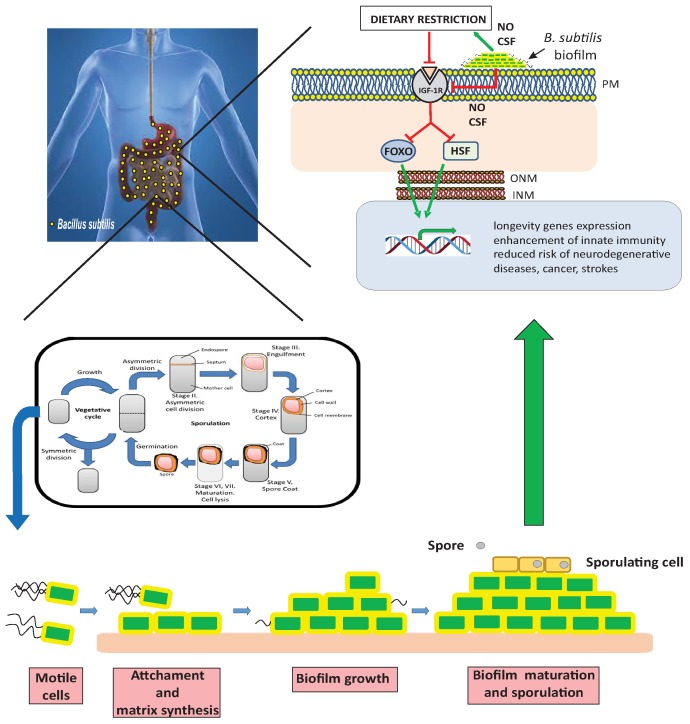
Figure [8]

Figure [9]
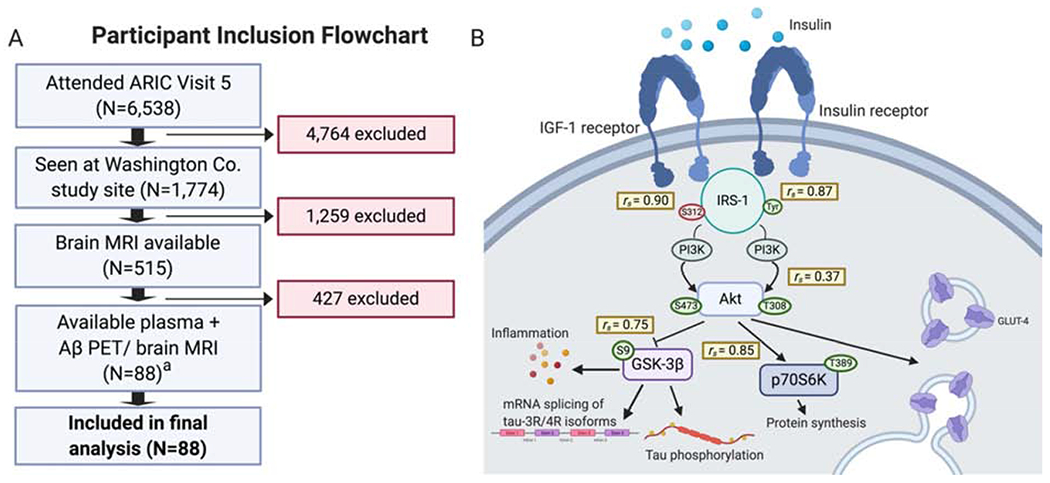
Figure [10]
Note: If you are interested in the full version of this target analysis report, or if you'd like to learn how our AI-powered BDE-Chem can design therapeutic molecules to interact with the INS target at a cost 90% lower than traditional approaches, please feel free to contact us at BD@silexon.ai.
More Common Targets
ABCB1 | ABCG2 | ACE2 | AHR | AKT1 | ALK | AR | ATM | BAX | BCL2 | BCL2L1 | BECN1 | BRAF | BRCA1 | CAMP | CASP3 | CASP9 | CCL5 | CCND1 | CD274 | CD4 | CD8A | CDH1 | CDKN1A | CDKN2A | CREB1 | CXCL8 | CXCR4 | DNMT1 | EGF | EGFR | EP300 | ERBB2 | EREG | ESR1 | EZH2 | FN1 | FOXO3 | HDAC9 | HGF | HMGB1 | HSP90AA1 | HSPA4 | HSPA5 | IDO1 | IFNA1 | IGF1 | IGF1R | IL17A | IL6 | INS | JUN | KRAS | MAPK1 | MAPK14 | MAPK3 | MAPK8 | MAPT | MCL1 | MDM2 | MET | MMP9 | MTOR | MYC | NFE2L2 | NLRP3 | NOTCH1 | PARP1 | PCNA | PDCD1 | PLK1 | PRKAA1 | PRKAA2 | PTEN | PTGS2 | PTK2 | RELA | SIRT1 | SLTM | SMAD4 | SOD1 | SQSTM1 | SRC | STAT1 | STAT3 | STAT5A | TAK1 | TERT | TLR4 | TNF | TP53 | TXN | VEGFA | YAP1

