TNF Target Analysis Report Summary


About the Target
Based on the provided context information, we can summarize the key viewpoints about TNF as follows:
TNF has the ability to prime and sensitize certain cells, such as fibroblast-like synoviocytes (FLS), by modifying their chromatin structure and inducing the expression of specific genes. This primed state can last for several days and results in hyperresponsiveness to interferons [1].
TNF can also induce cell tolerization in macrophages by triggering the expression of A20 and establishing a gene-specific chromatin barrier. This tolerized state reduces signaling input and prevents gene expression upon subsequent stimulation [1].
Anti-TNF antibodies are used in the treatment of inflammatory bowel diseases (IBD) and can act through different mechanisms, including blocking the actions of both soluble and membrane-bound TNF, interacting with TNF receptors, promoting antibody-dependent cellular cytotoxicity, and complement-dependent cytotoxicity [2].
During infection with Leishmania major, non-stimulated neutrophils produce low levels of TNF, which is associated with decreased cell degranulation and postponed cell death. However, treating neutrophils with ArtinM leads to their activation, increased TNF production, enhanced degranulation, and improved leishmanicidal capacity [3].
The duration of TNF exposure affects the balance between pro-survival NF-kappaB and pro-apoptotic caspase pathways. Brief exposure to TNF leads to weak NF-kappaB activation and stronger flux through the pro-apoptotic branch, promoting early apoptosis. Prolonged exposure to TNF sustains NF-kappaB activation, reducing pro-apoptotic flux and promoting cell survival [4].
TNF can regulate gene expression by modulating chromatin accessibility. Accessible chromatin enables rapid recruitment of nuclear factor kappaB (NFkappaB), while TNF-induced chromatin remodeling is required for the induction of genes with inaccessible chromatin. TNF can also induce the production of IFN-beta and interact with signal transducer and activator of transcription (STAT) signaling for optimal gene induction. Additionally, TNF can recruit co-activator proteins like IkappaBzeta and prevent gene induction by recruiting co-repressor complexes [5].
Overall, TNF plays a crucial role in cell priming and tolerization, inflammation, and cell survival/apoptosis. Additionally, TNF and its inhibitors have therapeutic implications for IBD and infectious diseases.
Based on the given context information, here is a comprehensive summary of the key viewpoints related to TNF (tumor necrosis factor):
Interaction and Signaling: There is an interaction between tumor necrosis factor and tumor necrosis factor receptor 2 signaling. This interaction involves up-regulated genes in SARS-CoV-2-infected cells, including cytokines and intracellular proteins [6]. Furthermore, TNF-alpha binds to TNF-alpha receptor 1 (TNFR1) and activates downstream signaling pathways, such as the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kappaB) pathway [7].
Role in Apoptotic Signaling: Tumor necrosis factor (TNF) plays a role in apoptotic signaling pathways. It can initiate the formation of Complex I/Death-Inducing Signaling Complex (DISC) and Complex II, involving various proteins such as FADD, procaspase-8/10, and cFLIP. Activation of these complexes can lead to apoptosis [7].
Immune Mechanisms and Arthritis: TNF has immune mechanisms induced by early-stage apoptotic cell infusion in arthritis. This includes the elimination of apoptotic cells by macrophages (MZMphi, splenic Mphi, peritoneal Mphi, and joint Mphi), migration of immune cells (Treg) to inflamed joints, and reprogramming of joint macrophages [8].
Treatment of Vitiligo: In patients with progressive vitiligo, the use of TNF-alpha inhibitors can halt depigmentation by inhibiting TNF-alpha effects on CD8+ T-cell activation and proliferation. However, in patients without vitiligo, inhibiting TNF-alpha can lead to the removal of the skin's innate protection against depigmentation and de novo vitiligo development [9].
Ligands and Receptors: TNF belongs to the TNF superfamily (TNFSF) and stimulates receptors of the TNF receptor superfamily (TNFRSF). TNFSF comprises various ligands (e.g., TNF, CD40L, CD95L) that interact with TNFRSF receptors, which can be categorized into death receptors, TRAF-interacting receptors, and decoy receptors based on their functional characteristics [10].
These viewpoints highlight the role of TNF in signaling, apoptosis, immune mechanisms, and disease conditions such as arthritis and vitiligo. TNF interacts with its receptors to modulate various cellular processes and can have both pro-inflammatory and anti-inflammatory effects depending on the context.
TNF, also known as tumor necrosis factor, plays a crucial role in inflammation and cellular processes. Before 24 hours of treatment, TNF activates the production of IL-8 through the NFkappaB signaling pathway [11]. This activation leads to the simultaneous production of reactive oxygen species (ROS) and the activation of antioxidant defenses. The JNK pathway also contributes to the activation of manganese superoxide dismutase (MnSOD) and TSPO transcription. ROS, acting as scavengers, induce the formation of TSPO polymers and increase TSPO turnover, which helps maintain mitochondrial integrity and regulate ROS production.
After 24 hours of TNF treatment, an overflow of antioxidant defenses occurs along with sustained TSPO turnover. This leads to the dysfunction of mitochondrial membrane complexes, which are responsible for the disruption of inner mitochondrial integrity. As a result, the apoptotic cascade is activated, ultimately leading to cell death. The time course of TNF-induced inflammation indicates two stages in this process [9].
To summarize, TNF activation initially stimulates IL-8 production and triggers antioxidant defenses, while also promoting TSPO turnover to maintain mitochondrial integrity and regulate ROS production. However, prolonged TNF treatment leads to an imbalance in antioxidant defenses and sustained TSPO turnover, causing disruption in mitochondrial membrane complexes and activation of apoptosis, ultimately resulting in cell death.
Figure [1]

Figure [2]

Figure [3]
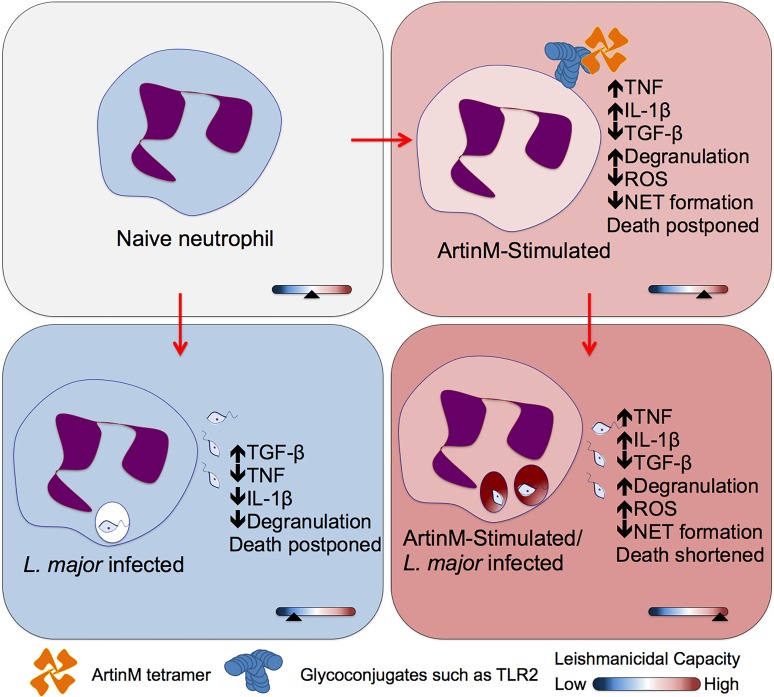
Figure [4]

Figure [5]
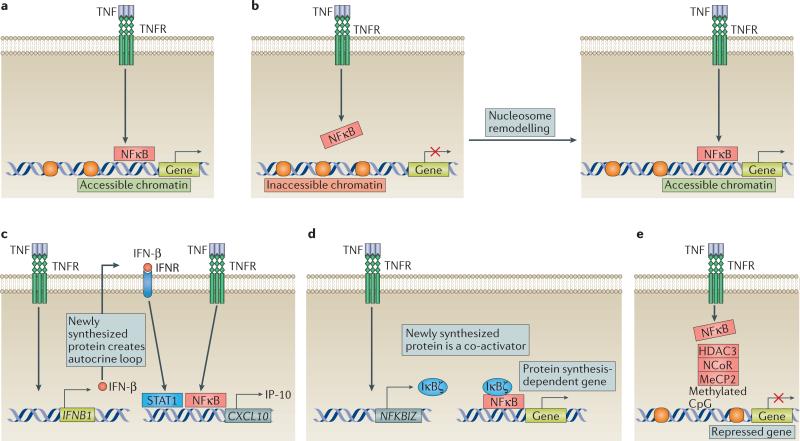
Figure [6]
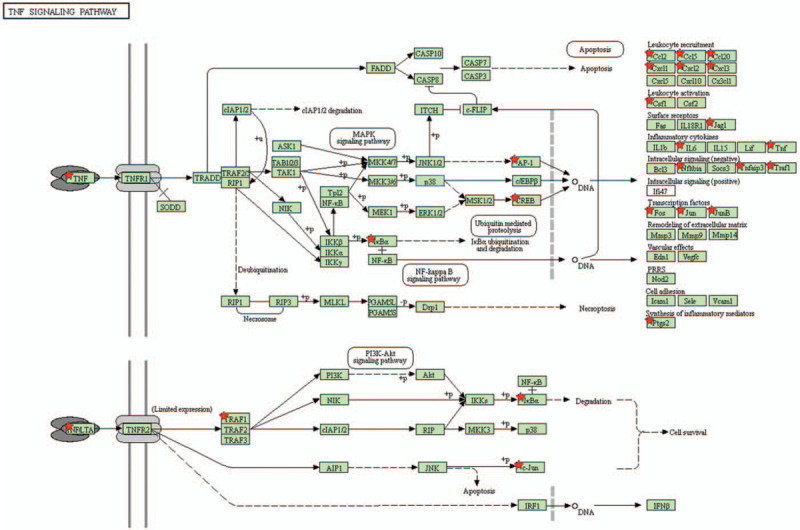
Figure [7]

Figure [8]
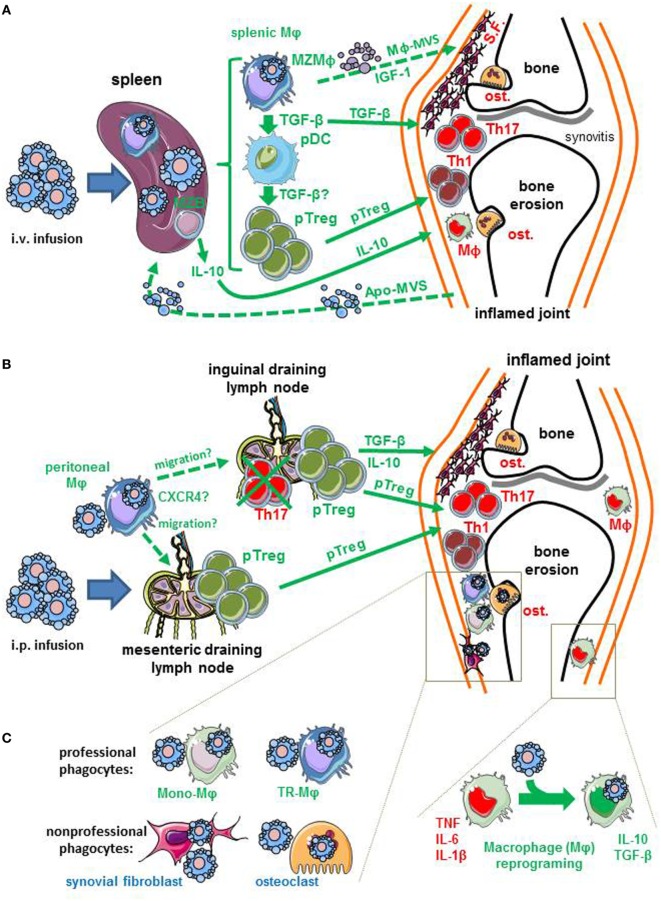
Figure [9]
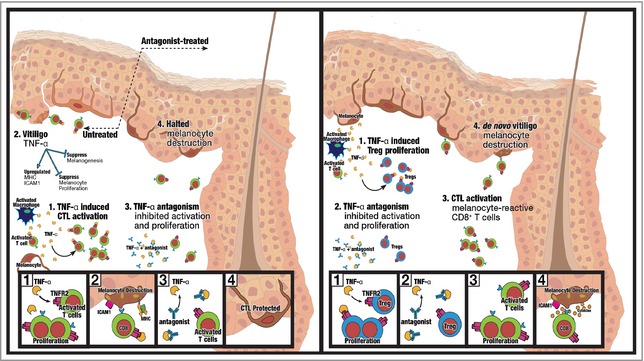
Figure [10]
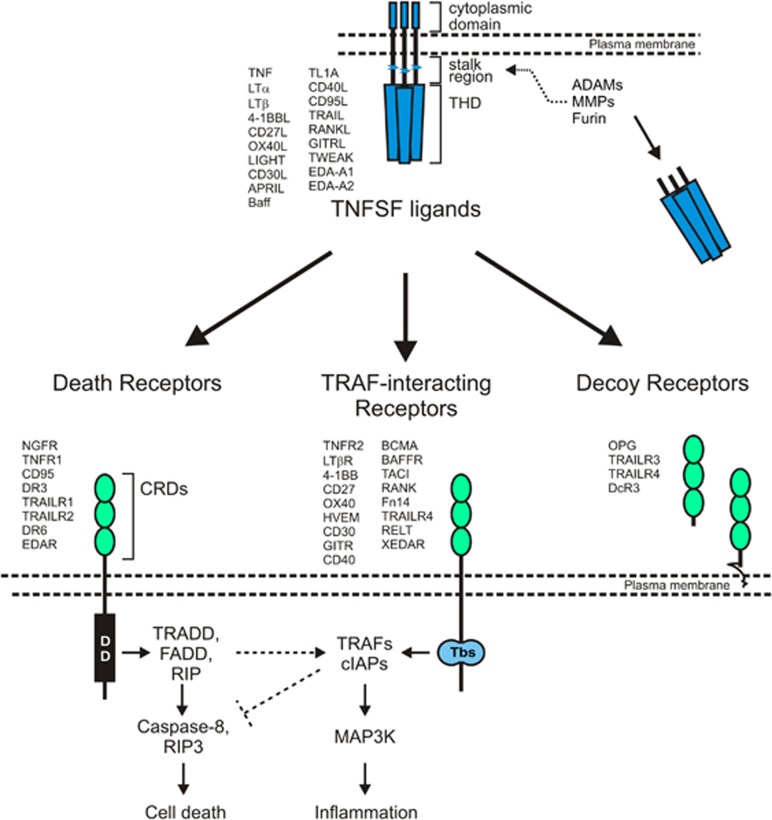
Figure [11]

Note: If you are interested in the full version of this target analysis report, or if you'd like to learn how our AI-powered BDE-Chem can design therapeutic molecules to interact with the TNF target at a cost 90% lower than traditional approaches, please feel free to contact us at BD@silexon.ai.
More Common Targets
ABCB1 | ABCG2 | ACE2 | AHR | AKT1 | ALK | AR | ATM | BAX | BCL2 | BCL2L1 | BECN1 | BRAF | BRCA1 | CAMP | CASP3 | CASP9 | CCL5 | CCND1 | CD274 | CD4 | CD8A | CDH1 | CDKN1A | CDKN2A | CREB1 | CXCL8 | CXCR4 | DNMT1 | EGF | EGFR | EP300 | ERBB2 | EREG | ESR1 | EZH2 | FN1 | FOXO3 | HDAC9 | HGF | HMGB1 | HSP90AA1 | HSPA4 | HSPA5 | IDO1 | IFNA1 | IGF1 | IGF1R | IL17A | IL6 | INS | JUN | KRAS | MAPK1 | MAPK14 | MAPK3 | MAPK8 | MAPT | MCL1 | MDM2 | MET | MMP9 | MTOR | MYC | NFE2L2 | NLRP3 | NOTCH1 | PARP1 | PCNA | PDCD1 | PLK1 | PRKAA1 | PRKAA2 | PTEN | PTGS2 | PTK2 | RELA | SIRT1 | SLTM | SMAD4 | SOD1 | SQSTM1 | SRC | STAT1 | STAT3 | STAT5A | TAK1 | TERT | TLR4 | TNF | TP53 | TXN | VEGFA | YAP1

