TP53 Target Analysis Report Summary


About the Target
The TP53 gene encodes the p53 protein, which plays a crucial role in cancer therapy. Various strategies can be used to target wild-type (wtp53) and mutant (mutp53) p53 proteins in cancer treatment. In cancers with wtp53, MDM2 inhibitors prevent p53 degradation and enhance its tumor-suppressive functions. For mutp53, specific p53 mutation targeting agents and inhibitors promote degradation or restore wild-type p53 functions. These strategies aim to induce tumor-suppressive responses such as cell cycle arrest, apoptosis, and senescence to inhibit tumor initiation and progression [1].
In HPV-driven carcinogenesis, the overexpression of E6 and E7 viral oncogenes leads to p53 and Rb degradation, resulting in deregulation of the cell cycle and oncogenes expression. This, combined with epigenetic and genetic modifications, can lead to the development of cancer. Additionally, microRNAs play a role in malignant transformation caused by HPVE6 and E7 expression in cervical cancer [2].
Certain thiol-binding small molecules can reactivate mutant p53 and induce oxidative stress and cell death. These compounds bind preferentially to specific cysteine residues in mutant p53, leading to thermostabilization and activation of p53 target genes. They can also react with cellular antioxidant components, further promoting oxidative stress and tumor cell death [3].
Therapeutic approaches targeting E3 ligases in the context of p53 show promise for both wild-type and mutant p53. In wild-type p53, antagonizing E3 ligases MDM2 and COP1 can stabilize and activate p53. In mutant p53, certain compounds can induce mutant p53 degradation by targeting E3 ligases directly or indirectly [4].
Puma, a target gene of p53, plays a critical role in mediating p53-induced apoptosis. Puma activates Bax/Bak, resulting in the release of cytochrome c and the induction of apoptosis. Loss of Puma function can hinder apoptosis [5].
In conclusion, strategies targeting TP53 aim to restore wild-type p53 functions in mutants or enhance the tumor-suppressive functions of wild-type p53. This can be achieved through various approaches such as MDM2 inhibitors, mutation-specific targeting agents, E3 ligase targeting compounds, and the activation of downstream targets like Puma. These strategies ultimately aim to induce tumor-suppressive responses and promote apoptosis in cancer cells.
Based on the given context information, here are some key viewpoints regarding the mechanism of action and therapeutic strategies of TP53 (p53):
Mechanism of action of PFD in the early stages of hepatocellular carcinoma (HCC):
- PFD activates the PPARgamma signaling pathway, which modifies the translocation of NF-kB p65/p50, preventing inflammation and HCC development. It also increases p53 activity and caspase 3 activation [6].
Therapeutic strategies for targeting mutant p53:
- Approaches can be classified into six categories: restoration of wild-type p53 activities, selective degradation of mutant p53, inhibition of protein-protein interactions involved in mediating gain-of-function of mutant p53, exploiting synthetic lethal vulnerabilities, inhibition of downstream survival pathways augmented by mutant p53, and immunotherapy based on the recognition of mutant p53 neoantigens [7].
Biphasic regulation of p53 under hypoxia:
- Under normoxia, p53 accumulation leads to senescence or apoptosis.
- Hypoxic conditions reduce p53 through lysosomal degradation, promoting cell survival and inducing autophagy.
- Prolonged hypoxia results in p53 stabilization, gene expression, and maintenance of cellular homeostasis [8].
Activation of SIRT1 deacetylase in CML and FLT3-ITD+ AML:
- BCR-ABL in CML and FLT3-ITD signaling in AML activate SIRT1 deacetylase downstream, leading to p53 pathway inhibition [9].
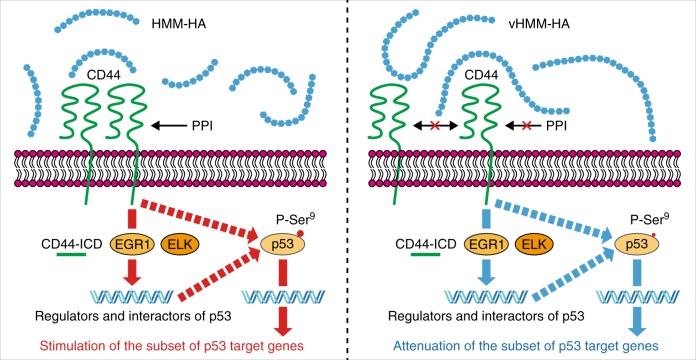
Effects of vHMM-HA on p53 pathway:
- vHMM-HA attenuates CD44 protein-protein interactions (PPI) and CD44-dependent gene expression.
- It partially attenuates p53 and its target genes, offering a p53-dependent cytoprotective effect [10].
These viewpoints provide insights into different aspects of p53, including its role in HCC, therapeutic strategies for mutant p53, regulation of p53 under hypoxia, effects on the p53 pathway in specific cancers, and modulation of p53-dependent cellular processes by vHMM-HA.
Figure [1]
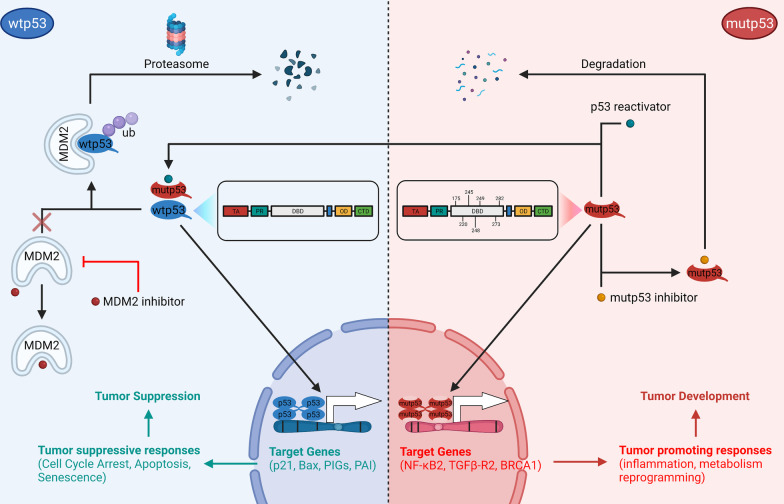
Figure [2]
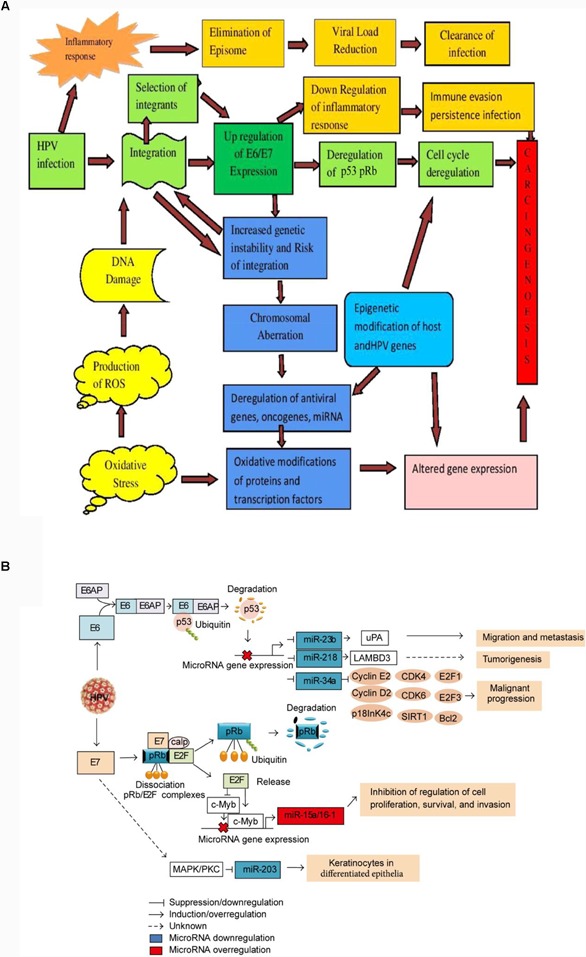
Figure [3]
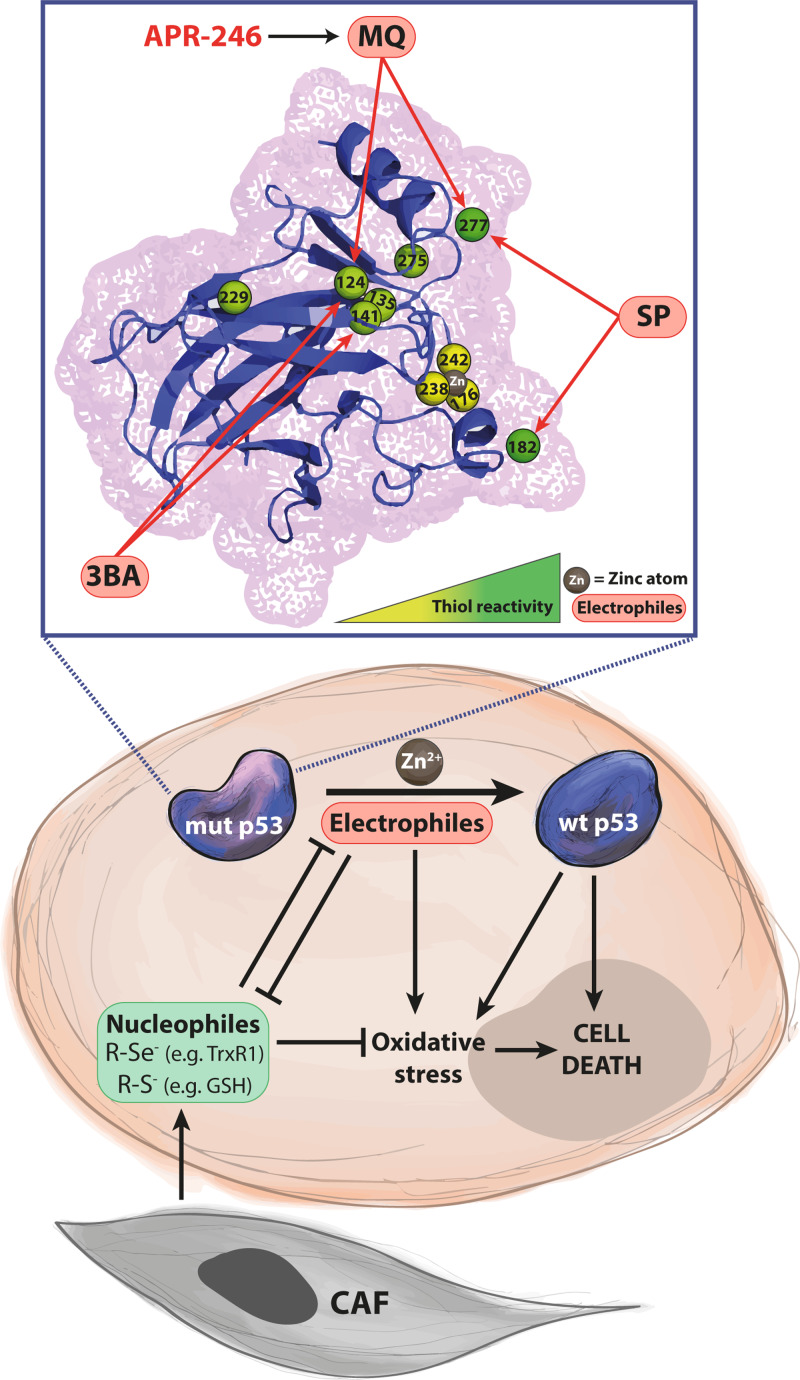
Figure [4]

Figure [5]
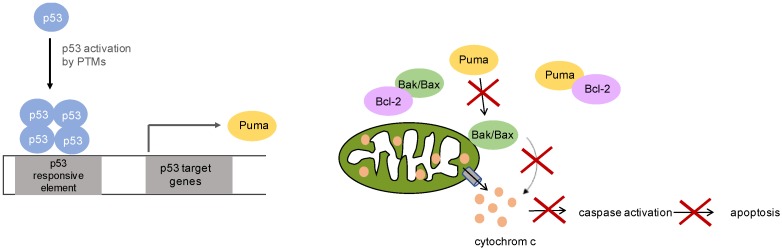
Figure [6]
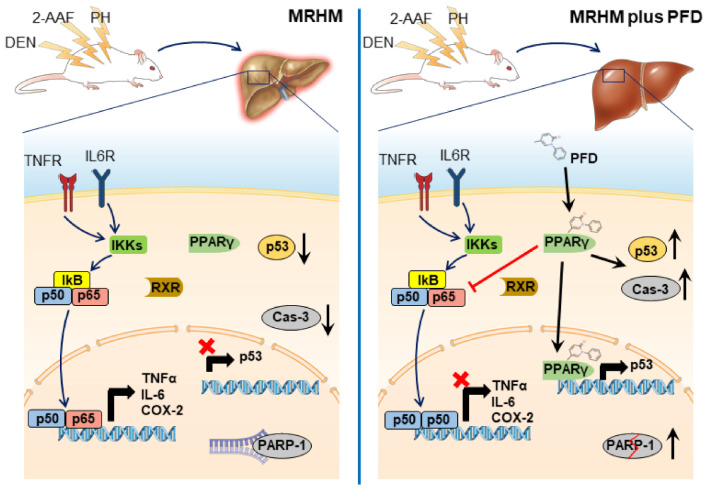
Figure [7]
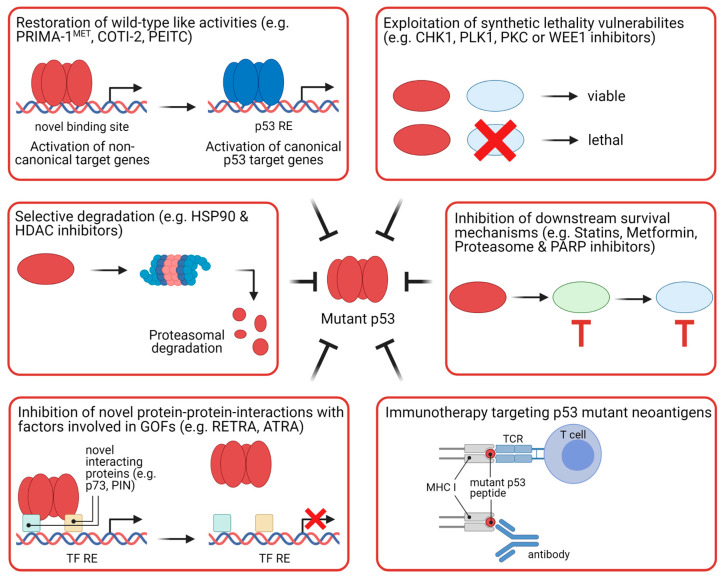
Figure [8]
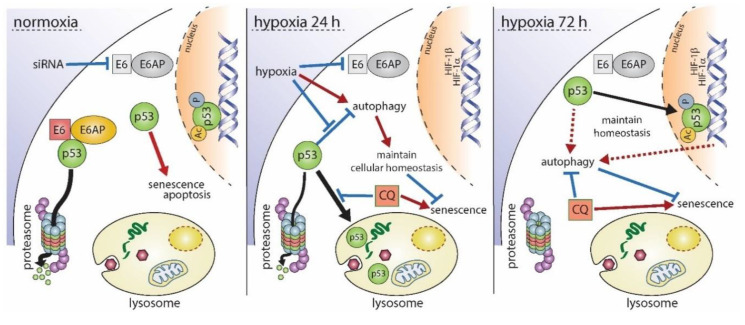
Figure [9]
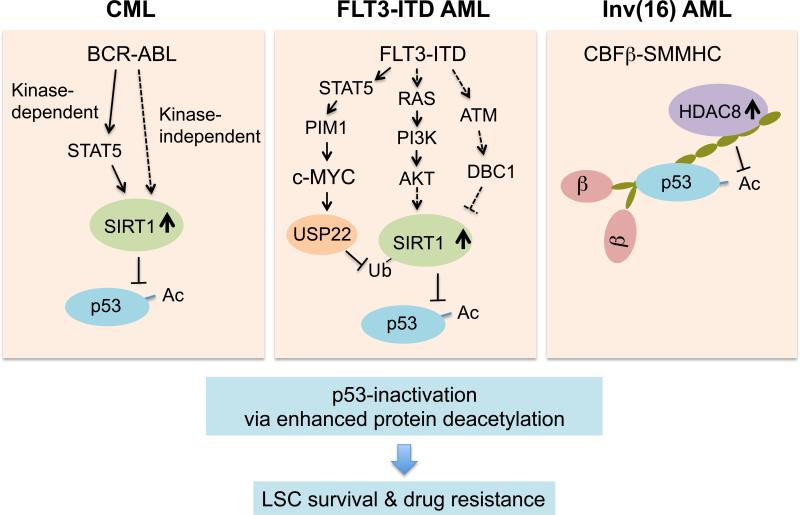
Figure [10]
Note: If you are interested in the full version of this target analysis report, or if you'd like to learn how our AI-powered BDE-Chem can design therapeutic molecules to interact with the TP53 target at a cost 90% lower than traditional approaches, please feel free to contact us at BD@silexon.ai.
More Common Targets
ABCB1 | ABCG2 | ACE2 | AHR | AKT1 | ALK | AR | ATM | BAX | BCL2 | BCL2L1 | BECN1 | BRAF | BRCA1 | CAMP | CASP3 | CASP9 | CCL5 | CCND1 | CD274 | CD4 | CD8A | CDH1 | CDKN1A | CDKN2A | CREB1 | CXCL8 | CXCR4 | DNMT1 | EGF | EGFR | EP300 | ERBB2 | EREG | ESR1 | EZH2 | FN1 | FOXO3 | HDAC9 | HGF | HMGB1 | HSP90AA1 | HSPA4 | HSPA5 | IDO1 | IFNA1 | IGF1 | IGF1R | IL17A | IL6 | INS | JUN | KRAS | MAPK1 | MAPK14 | MAPK3 | MAPK8 | MAPT | MCL1 | MDM2 | MET | MMP9 | MTOR | MYC | NFE2L2 | NLRP3 | NOTCH1 | PARP1 | PCNA | PDCD1 | PLK1 | PRKAA1 | PRKAA2 | PTEN | PTGS2 | PTK2 | RELA | SIRT1 | SLTM | SMAD4 | SOD1 | SQSTM1 | SRC | STAT1 | STAT3 | STAT5A | TAK1 | TERT | TLR4 | TNF | TP53 | TXN | VEGFA | YAP1

